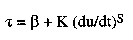
Allan Holmgren and Willis Forsling
Dept. of Chemical and Metallurgical Engineering
Div. of Inorganic Chemistry
Luleå University of Technology
971 87 Luleå.
Purpose
The purpose of this prestudy was to collect information about the compounds used to form aircraft de/anti-icing fluids and especially to examine these fluids with respect to their recycling properties.
Background information
Any contamination of aircraft surfaces, e.g. ice contamination, is considered as a serious threat to safe climb and maneuvering capabilities. If the aircraft surface is contaminated, the prescribed aerodynamic safety margins specified for the aircraft with respect to acceleration and climbing performance may be seriously reduced or completely eliminated. Therefore the clean aircraft concept is worldwide accepted implying that e.g. ice and snow have to be removed from the surface of the aircraft before take off. Aircraft icing conditions can be expected when air temperatures fall below the freezing point, and when moisture or ice occurs in the form of either precipitation or condensation.
To accomplish a surface free from ice, frost or snow, warm fluids based on mixtures of glycol and water is generally used. In addition to glycols and water, these fluids contain small amounts of anionic and/or non-ionic wetting agents, corrosion inhibitors, oxidation stabilizators and pH modifiers. A formulation containing all these compounds and at least 80 % by weight of glycol is called a Type 1 fluid. If the glycol content is minimum 50 % by weight and the fluid also contains a pseudo plastic thickening system it is called a Type 2 fluid [1,2].
The thickener system makes the two fluid types differ in physical properties. At the same temperature and shear rate Type 1 fluids have considerably lower viscosity than Type 2 fluids and demonstrate Newtonian characteristics i.e. the viscosity is constant and not shear-rate dependent. The higher viscosity of a Type 2 fluid implies a thicker film on the aircraft surface and thereby an increased holdover time i.e. the period of time when ice or snow is prevented from adhering to the surface. The holdover time varies depending on the weather condition. Snow and a temperature below -7 °C implies an achieved holdover time of 6-15 minutes for a Type 1 fluid mixture while the corresponding figures for a Type 2 fluid is 20-45 minutes.
Type 2 is predominantly an anti-icing agent because it persists in the presence of snow and other forms of freezing precipitation. If the wing therefore already has ice or snow on it, this is usually first cleaned off using Type 1 fluid.
Type 2 fluids are non-Newtonian liquids exhibiting pseudoplastic flow behaviour, which means that the viscosity is shear-rate dependent and they have measurable yield values. The viscosity of the fluid mixture decreases with increasing shear force implying the de/anti-icing fluid to flow off the aircraft at take-off [3].
The two types of fluid also differ in their sensitivity to high temperature, contamination and mechanical shearing. Temperatures above 90 °C will destroy the thickener and therefore cause a reduced viscosity. Likewise, pumping the fluid through narrow valve openings or using centrifugal pumps or high-speed piston pumps will cause advers mechanical shearing and hence a reduction in the viscosity. Screw pumps or diaphragm pumps and low pumping speeds are recommended. Contaminations like Type 1 fluid, dyes or inorganic salts may also reduce the viscosity of Type 2 fluids. Since the high viscosity or more specifically the yield value of the fluid contributes to the holdover time it is important to avoid high temperatures and mechanical shearing if a Type 2 fluid mixture shall meet the specifications [4]. In this context it should be mentioned that the de/anti-icing fluids used have been thoroughly tested with regards to deicing capability, holdover time and flow-off properties according to standards outlined by the Association of European Airlines (AEA) or International Standards Organisation (ISO).
Application equipment
Most of the equipment used today are trucks with a chassis on which the fluid tanks, pumps and heating facilities are installed. Modern trucks are accomodated to the degradation sensitive Type 2 fluids and therefore equipped with cavity pumps or diaphragm pumps. The application of the de/anti-icing fluid mixture may take place at boarding gates, remote facilities, or near the ends of departure runways. From both environmental aspects and a fluid recycling point of view the many locations may be a disadvantage, since it makes the collection of the waste fluid much more difficult. For that reason a stationary system seems to be more appropriate.
Stationary de/anti-icing systems are in use at a few locations such as the airports of Luleå in Sweden, Oslo in Norway and at the new Munich airport in Germany. As a part of our preinvestigation we made study tours both to Kallax airport in Luleå and to Frans Josef Straus airport in Munich.
The stationary systems are operated by the aid of a computer and consists of a gantry with spraying nozzles moving over the aircraft. As the computer controls the de-icing process, working errors are practically excluded and a consistent de-icing quality can be ensured. It may be noted that the full resposibility of accepting the performed treatment lies with the pilot in command. In addition to the gantry the systems also include a de-icing apron and a recovery plant.
The obvious advantage of such a system is that excess fluid is restricted to a specially designed concrete apron from which it is collected and pumped to a recovery plant. Furthermore, the computer control assures that no more glycol enters the environment than needed for an effective deicing.
Considering the publics environmental awareness and growing concern about environmental pollution, these advantages should not be underestimated.
The major drawback with the gantry system today is its inability to work with Type 2 fluids. Due to the spraying nozzles the thickener is partly destroyed with a resulting undesirable reduction in viscosity. A parallel spray system with appropriately adjusted flow rates and nozzle performance would eliminate that drawback.
Environmental aspects
At European airports, the glycols used for de/anti-icing purposes are either di-ethylene glycol (DEG) or mono-propylene glycol (MPG). These two compounds are entirely biodegradable which means that microorganisms under aerobic conditions convert the glycols to carbon dioxide and water. However, the rate of degration varies. Although the rate increases with temperature and access to oxygen, di-ethylene glycol seems to be less readily biodegradable than propylene glycol.
When it comes to the toxicity of the two compounds the situation is somewhat confusing [2]. There is a general agreement about the non-toxicity of propylene glycol. A pure quality of this compound is even used in the pharmaceutical industry and no organ has yet been identified as a target for oral lethal effects.
According to the regulations used in the U.S.A., DEG has a high order of toxicity and the human lethal oral dose is put to 1.0 ml/kg.
However, there is also reports showing DEG should be classified to be equally toxic as glycerol, and glycerol is considered to be a non-toxic compound.
In any case, for safety reasons it seems reasonable to classify DEG as a toxic compound as long as the situation about its toxicity is unclear.
Recycling of de/anti-icing fluids
An interesting question concerning de/anti-icing fluids is: What to do with the fluids once they have been used?
The waste fluid can be drained into surface waters and biodegraded which implies oxygen consumption or be collected and treated in waste water treatment plants. The thickener of Type 2 fluids is considered to have no negative effects on the environment, although it is a synthetic polymer, but may have like other additives a negative effect on the biodegradability. Other possibilities are destruction by burning and recycling.
Considering the possibility that the aviation industry in the future may face more stringent environmental regulation of glycols and additives, recycling should be the most attractive procedure, and is already employed for Type 1 fluids at the airports in Munich, Oslo and Luleå.
Either of two recovery principals may be discernible. The waste fluid may be processed in the recovery system with the aim to preserve as much as possible of the expensive additives or the additives may be completely separated from the glycol/water mixture before distillation of the mixture. At our visit to Munich we discussed these two principals with representatives from Hoechst Chemicals, a company that, among other products, delivers de/anti-icing fluids. Both Dr. Peter Geymayer and Dr. Klaus Pöllmann at the department of research and development insisted on the necessity of removing the additives before the distillation of the glycol/water mixture. Their opinion may be governed by commercial considerations since the additives are rather expansive. However, considering the degradation sensitivity of Type 2 fluids it seems reasonable to remove at least the thickener before the waste fluid is distilled.
The recovery systems currently in use consists of a number of cleaning steps. In principal the recycling construction is similar. The waste fluid first passes through two or three filters with a pore size varying between 50 µm and 5 µm, and the through a humic-filter, a cationic exchanger and finally through an anionic exchanger. Then water is evaporated in a distillation tower. After that the recycled glycol has to be reformulated and subjected to careful quality control to ensure compliance with a Type 1 fluid. So far only Type 1 fluids are recovered.
Prestudy design
The information about de/anti-icing fluids and their recovery described in the previous sections was obtained from data sheets, brochures, discussions with representatives from the De-icing company, and a literature search giving 210 references. The literature search comprised the data bases; Compendix, ASTIS and Chemical Abstract. According to these references including a BASF patent application from 1989, a Type 2 fluid contains in addition to glycol ( DEG and/or MPG ) and water also small amounts of wetting agents, corrosion inhibitors, oxidation stabilizors, pH adjustors and a thickening agent [5]. The thickener is a polymer forming a cross-linked network and gives the system a pseudoplastic flow behaviour. This means that the viscosity of the fluid is shear-rate dependent and the flow can be described by a general expression of the form,
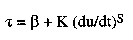
where 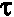 is the shear stress,
is the shear stress, 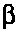 is the yield value, K is the plastic viscosity
coefficient, du/dt is the shear rate, and S is the shear rate index. The first
term in this expression primarely contributes to the holdover time while the
flow properties are mainly governed by the second term. From a recycling point
of view it is important to know the properties of the particles constituting
the thickening system. However, at the Hoechst company they apparently regarded
this information as confidential. Moreover no patent application of their new
Type 2 fluid existed, that could give us this information. However, a patent
application from 1987 may be helpful [6]. It tells that the fluid, in addition to
DEG ( 40.00% ), water ( 49.4% ), and 1,2-propanediol ( 10.00% ) also may
contain 78:20:2 acrylamide - Na acrylate - crosslinker copolymer ( 0.03% ),
crosslinked polyacrylic acid ( or metal salt ) ( 0.20% ), sodium
(C15-alkyl)benzenesulfonate ( 0.15% ), benzotriazole ( 0.03% ), triethanolamine
( 0.05% ), NaOH ( 0.03% ), and KOH ( 0.11% ). In this mixture the crosslinked
polyacrylate constitutes the thickening system, but the crosslinker used is not
mentioned in the patent application. The mixture had high-humidity test
holdover time of more than 8 hours and freezing-rain endurance test hold-over
time of 37 minutes. The viscosity at 20, 0, -10, -25, and -35 °C was
9, 15, 20, 30, and 45 Pas, respectively. In this context it should also be
mentioned that during the current project period, the anionic surfactant in at
least Type 1 fluids from Hoechst was substituted for a nonionic surfactant. The
ionic surfactant caused problems with the anion exchanger because of strong
adsorption.
is the yield value, K is the plastic viscosity
coefficient, du/dt is the shear rate, and S is the shear rate index. The first
term in this expression primarely contributes to the holdover time while the
flow properties are mainly governed by the second term. From a recycling point
of view it is important to know the properties of the particles constituting
the thickening system. However, at the Hoechst company they apparently regarded
this information as confidential. Moreover no patent application of their new
Type 2 fluid existed, that could give us this information. However, a patent
application from 1987 may be helpful [6]. It tells that the fluid, in addition to
DEG ( 40.00% ), water ( 49.4% ), and 1,2-propanediol ( 10.00% ) also may
contain 78:20:2 acrylamide - Na acrylate - crosslinker copolymer ( 0.03% ),
crosslinked polyacrylic acid ( or metal salt ) ( 0.20% ), sodium
(C15-alkyl)benzenesulfonate ( 0.15% ), benzotriazole ( 0.03% ), triethanolamine
( 0.05% ), NaOH ( 0.03% ), and KOH ( 0.11% ). In this mixture the crosslinked
polyacrylate constitutes the thickening system, but the crosslinker used is not
mentioned in the patent application. The mixture had high-humidity test
holdover time of more than 8 hours and freezing-rain endurance test hold-over
time of 37 minutes. The viscosity at 20, 0, -10, -25, and -35 °C was
9, 15, 20, 30, and 45 Pas, respectively. In this context it should also be
mentioned that during the current project period, the anionic surfactant in at
least Type 1 fluids from Hoechst was substituted for a nonionic surfactant. The
ionic surfactant caused problems with the anion exchanger because of strong
adsorption.
Based on expected problems with the recycling of Type 2 fluids, we designed some initial experiments in order to get some basic data indicating how the thickener may be removed from the fluid and also how the time between regeneration of the anion exchangers may be increased. The following experiments were performed:
1. Adsorption of a Type 2 fluid ( LTV 1704 ) on aluminium oxide (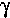 -
-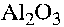 ),
wollastonite (
),
wollastonite (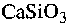 ) and adsorption filters consisting of a polymer matrix
and attached non-ionic groups ( Lewatite OC 1064 ) and cationic groups ( IRA 458 ).
) and adsorption filters consisting of a polymer matrix
and attached non-ionic groups ( Lewatite OC 1064 ) and cationic groups ( IRA 458 ).
2. Adsorption of anionic surfactants ( sodium oleate and sodium dodecylsulphate ) on Lewatite OC 1064 and IRA 458.
3. Flocculation of LTV 1704 using aqueous solutions of 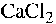 and
and 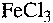 .
.
4. Adsorption of glycerol on 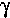 -
-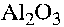 .
.
To identify the species adsorbed or flocculated we used Fourier Transform
Infrared Spectroscopy ( FTIR ) and FT - Raman spectroscopy. The use of
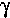 -
-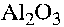 is justified due to the fact that all aluminium objects are covered with
a thin layer of alumina.
is justified due to the fact that all aluminium objects are covered with
a thin layer of alumina.
Experimental
Type 2 fluid, the adsorption filter Lewatite ( OC 1064 ), and the anion exchanger IRA 458 was obtained from Hoechst Chemicals, Germany. Sodium oleate was obtained from BDH Chemicals Ltd., England, and sodium dodecylsulphate of technical grade was obtained from Merck. High purity alumina grade from Mandoval Ltd., England and calcium silicate ( Aldrich 99% ) were used.
In the flocculation experiments, aqueous solutions of 0.1 M 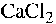 and 0.1 M
and 0.1 M
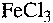 were used. The flocculated phase was collected using a Munktell micropore
filter ( OOH ), from Grycksbo, Sweeden.
were used. The flocculated phase was collected using a Munktell micropore
filter ( OOH ), from Grycksbo, Sweeden.
Samples adsorbed on aluminium oxide and wollastonite were dried at ambient temperature before the spectra were recorded. The flocculated samples were dried at 100 °C for two hours. All samples were washed with water before spectra were recorded.
Raman and IR spectra were recorded using a Perkin-Elmer PE 1760X Fourier transform infrared ( FTIR ) spectrometer equipped with a near-infrared ( NIR ) Raman bench. Data manipulation was carried out using a software package ( Lab Calc ) from the Galactic Industries Corporation.
For the IR measurements, spectra were collected by the diffuse reflectance method, commonly known as DRIFT when used in conjunction with FT-IR instruments. The samples were diluted with dry powderized potassium bromide. Fifty scans were combined, and the resultant interferogram was Fourier transformed to obtain a resolution of 4 (1/cm) over the spectral range. All DRIFT spectra were converted to Kubelka-Munk units [7].
In the Raman measurements, the excitation source was a Spectron SL 301 neodymium-doped yttrium aluminium garnet ( Nd:YAG ) laser system providing intensity-stabilized emission at 1064 nm. The scattered light was collected with a 180 backscattering-geometry lens. All Raman spectra were recorded at 4 (1/cm) resolution using typically 200 accumulations.
Results and Discussion
Adsorption of Type 2 fluid:
The DRIFT spectrum and the NIR FT-Raman spectrum of Type 2 fluid (
LTV1704 ) on 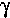 -
-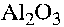 are shown in figures 1 and 2, respectively. In these
spectra the background spectrum of
are shown in figures 1 and 2, respectively. In these
spectra the background spectrum of 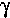 -
-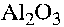 has been subtracted. Furthermore, the
sample was thoroughly washed with water and subsequently dried in order to
reduce the influence of DEG and water on the spectrum. As evident from the
infrared spectrum ( Fig.1 ) the sample still contains water and DEG. The
absorption bands at 3400 (1/cm) and 1660 cm-1 both
indicate hydrogen bonded OH functions. The former frequency originates from OH
stretching vibrations while the latter frequency is due to the bending
vibration of water. The absorption frequency at 1040 (1/cm) is typical
for a primary C-OH group and the absorption at 1127 cm-1 and 1064
(1/cm) belong to the antisymmmetric stretch and the symmetric stretch
of the C-O-C entity in DEG [8].
has been subtracted. Furthermore, the
sample was thoroughly washed with water and subsequently dried in order to
reduce the influence of DEG and water on the spectrum. As evident from the
infrared spectrum ( Fig.1 ) the sample still contains water and DEG. The
absorption bands at 3400 (1/cm) and 1660 cm-1 both
indicate hydrogen bonded OH functions. The former frequency originates from OH
stretching vibrations while the latter frequency is due to the bending
vibration of water. The absorption frequency at 1040 (1/cm) is typical
for a primary C-OH group and the absorption at 1127 cm-1 and 1064
(1/cm) belong to the antisymmmetric stretch and the symmetric stretch
of the C-O-C entity in DEG [8].
However, in addition to the mentioned absorption frequencies we also observe two infrared bands at 1570 (1/cm) and 1408 cm-1 which neither originate from water nor DEG. These two frequencies are typical for the antisymmetric and symmetric stretching vibration of a carboxylate group, the higher frequency belonging to the antisymmetric mode. Furthermore, the absorbtion bands at 1215 (1/cm) and 1180 (1/cm) , might originate from the antisymmetric stretching vibration of the sulfonate group in sodium alkylbenzene sulfonate. The local symmetry of the free sulfonate group is C(3v), implying a doubly degenerate antisymmetric mode [9]. Therefore, if the degeneracy of the mode is removed by e.g. strong interaction with a counterion, two absorption bands would be expected.
In the C-H stretching region, viz. 3100 - 2800 (1/cm), the infrared
spectrum shows absorption bands at 2960, 2926, and 2857 (1/cm),
corresponding to the antisymmetric 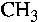 stretch, the antisymmetric
stretch, the antisymmetric 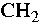 stretch,
and the symmetric
stretch,
and the symmetric 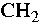 stretch, respectively. The symmetric
stretch, respectively. The symmetric 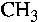 stretching
vibration is not resolved in our infrared spectrum. The
stretching
vibration is not resolved in our infrared spectrum. The 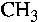 group may originate
either from 1,2-propanediol or from the end terminal group of the alkyl chain
in alkylbenzene sulfonate. The peak frequency at 1460 (1/cm) is
assigned to the symmetric methylene bending vibration.
group may originate
either from 1,2-propanediol or from the end terminal group of the alkyl chain
in alkylbenzene sulfonate. The peak frequency at 1460 (1/cm) is
assigned to the symmetric methylene bending vibration.
The Raman spectrum of LTV1704 adsorbed on 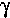 -
-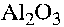 is shown in figure 2. The most
dominant lines in the spectrum are caused by methyl groups and the methylene
groups. The symmetric stretch of the methyl groups, which could not be
identified in the infrared spectrum, gives rise to the peak frequency at 2883
cm-1 and the antisymmetric stretch of the methylenes appears at 2856
(1/cm). The symmetric bending vibration of the methylene groups at
1451 (1/cm) appears as a resolved doublet caused by the antisymmetric
bending of methyl groups at 1441 (1/cm). It is interesting to note
that the Raman spectrum reveals the existence of an aromatic or unsaturated
structure. The lines at 3062 (1/cm) and 1601 (1/cm) are a
strong evidence.
is shown in figure 2. The most
dominant lines in the spectrum are caused by methyl groups and the methylene
groups. The symmetric stretch of the methyl groups, which could not be
identified in the infrared spectrum, gives rise to the peak frequency at 2883
cm-1 and the antisymmetric stretch of the methylenes appears at 2856
(1/cm). The symmetric bending vibration of the methylene groups at
1451 (1/cm) appears as a resolved doublet caused by the antisymmetric
bending of methyl groups at 1441 (1/cm). It is interesting to note
that the Raman spectrum reveals the existence of an aromatic or unsaturated
structure. The lines at 3062 (1/cm) and 1601 (1/cm) are a
strong evidence.
From the spectral interpretations above, one important conclusion emerges. They
show that it is possible to separate some additives, e.g. the thickener, from
the glycol-water based deicing fluid using 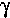 -
-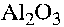 . The thickener is associated
with the carboxylate groups indicated.
. The thickener is associated
with the carboxylate groups indicated.
To compare these results with adsorption on other substrates, we substituted
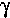 -
-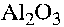 for wollastonite, Lewatite and the strongly basic anion exchanger IRA
458. Lewatite consists of a polystyrene matrix onto which nonionic groups are
attached and IRA 458 is a cross-linked acrylic-divinylbenzene resin onto which
for wollastonite, Lewatite and the strongly basic anion exchanger IRA
458. Lewatite consists of a polystyrene matrix onto which nonionic groups are
attached and IRA 458 is a cross-linked acrylic-divinylbenzene resin onto which
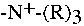 groups are bounded.
groups are bounded.
The infrared spectrum of the mineral wollastonite shows strong absorption below 1200 (1/cm), implying that no adsorbed molecules could be detected in this spectral region. However, absorption bands at 2927 (1/cm), 2856 (1/cm), 1560 (1/cm), 1458 (1/cm) and 1420 (1/cm) indicate that the same types of chemical structures are adsorbed on wollastonite as on aluminium oxide ( fig.3). The main difference between the spectrum of the species adsorbed on the two substrates is the lower infrared intensities of the bands belonging to the species adsorbed on wollastonite. This may be due to a lower surface area of the mineral or to its lower adsorption ability.
The infrared spectrum of adsorbed species on Lewatite is shown in figure 4, where the spectrum of Lewatite has been subtracted from the spectrum of Lewatite treated with Type 2 fluid. It may be noted that Lewatite exhibits many strong bands in almost the entire wavenumber region between 3100 (1/cm) and 400 (1/cm). Therefore, the difference spectrum in figure 4 must be interpreted with some care. However, it is evident that the spectrum shows no bands at about 1560 (1/cm) and 1410 (1/cm). These bands appear in spectra of adsorbed species on both aluminium oxide and wollastonite and were assumed to indicate the presence of a thickener system. The absorption bands at about 1130 (1/cm) and 1070 (1/cm) belong to adsorbed glycol, although the sample had been thoroughly washed with water before the spectrum was recorded.
The anion exchanger, IRA458, is a macroporous ( or macroreticular ) polymer having large-size channels, which offers the ions easy access to the functional groups of the exchanger. An average pore diameter of 1300 Å is not unusual. Therefore, IRA458 should be very effective in adsorbing additives from the Type 2 fluid. Unfortunately the anion exchanger exhibits strong infrared absorptions in the region below 1650 (1/cm), which omitted any unambiguous subtraction of the resin from the spectrum of the resin treated with Type 2 fluid.
Adsorption of anionic surfactants:
The strong adsorption of wetting agents like anionic surfactants by anion exchangers has caused problems with the lifetime of the exchanger. The exchanger became blocked, and had to be regenerated far too often. To elucidate the adsorption behaviour of anionic surfactants we choosed a non-ionic resin ( Lewatite ). A non-ionic resin should be a less effective adsorber than a polymer resin with cationic groups.
According to the infrared spectrum shown in figure 5, sodium oleate is adsorbed
on Lewatite. The absorption band at 3004 (1/cm) is characteristic of a
C-H stretch of the HC=CH entity at the cis 9 position in the acyl chain. The
bands at 2926 (1/cm) and 2852 (1/cm) are the antisymmetric
and symmetric stretch of the methylene groups. Some of the oleate molecules
form oleic acid as evident from the carbonyl band at 1709 (1/cm). The
bands at 1567 (1/cm) and at about 1416 (1/cm) are assigned to
the antisymmetric and symmetric stretching vibration of the carboxylate group.
On the lowfrequency side of the latter band a weak band appears, which probably
is due to the bending vibration of the 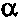 -
-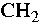 groups. It may be noted that the
sample containing adsorbed sodium oleate had been thoroughly washed with water
before the spectrum was recorded. Not even a 1.0 M NaOH(aq) solution was able
to completely remove adsorbed sodium oleate. This indicates that the ion
exchanger must be hard to completely regenerate and that the exchange capacity
is lower after a regeneration than for an unused adsorption resin.
groups. It may be noted that the
sample containing adsorbed sodium oleate had been thoroughly washed with water
before the spectrum was recorded. Not even a 1.0 M NaOH(aq) solution was able
to completely remove adsorbed sodium oleate. This indicates that the ion
exchanger must be hard to completely regenerate and that the exchange capacity
is lower after a regeneration than for an unused adsorption resin.
Also sodium dodecylsulfate is strongly adsorbed on Lewatite. This is shown in figure 6, where the strong infrared absorption band centered at about 1240 (1/cm), is due to the sulfate group. Like sodium oleate, SDS could not be completely removed using a 1.0 M aqueous solution of sodium hydroxide. The strong adsorbtion of anionic surfactants by Lewatite indicates that a polymer resin with attached cationic groups should be even more difficult to regenerate. The natural choice would be to use non-ionic wetting agents in order to enhance the life-time of an anion exchanger. In this context it may be noted that the Hoechst company substituted their anionic wetting agent for a non-ionic surfactant during our project period.
Flocculation experiments:
The thickening system may be regarded as negatively charged colloidal
particles, which are stabilized below a certain ionic strength or within a
certain pH interval. One way to separate the thickening system from the deicing
fluid is to destabilize the particles and make them flocculate or coagulate.
This may be effectuated by small amounts of added electrolyte. When the
doublelayer repulsive interaction of the particles is sufficiently reduced they
will approach each other close enough for the van der Waals forces to
predominate and the particles will flocculate [10]. According to the Schulze-Hardy
rule, an electrolyte is more effective the higher its counter-ion charge.
Therefore, 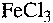 is more effective than
is more effective than 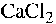 to induce flocculation. In
separate flocculation experiments we added both these electrolytes to Type 2
fluid. As expected the particles flocculated and could be separated from the
fluid by filtration. The same experiments were performed using a Type 1 fluid.
In this case no flocculation was observed. Since the main difference between
the two types of fluid is the thickening system, the flocs should contain the
thickener and possibly also some of the additives. The infrared spectrum of the
floc separated from Type 2 fluid, is shown in figure 7. Before the spectrum was
recorded the floc was washed with water and subsequently dried at 100 °C for two
hours. The most dominant spectral features are the OH vibrations in the
hydrogen bonding region ( around 3380 (1/cm)), the methylene
stretching vibrations at 2928 (1/cm) and 2857 (1/cm), and the
carboxylate modes at 1564 (1/cm)and 1413 (1/cm). These
vibrational modes are also evident in the spectrum of LTV1704 adsorbed on
aluminium oxide, and should originate from the thickener. To remove the
thickener from Type 2 fluids, it would therefore be possible to flocculate the
thickener using e.g.
to induce flocculation. In
separate flocculation experiments we added both these electrolytes to Type 2
fluid. As expected the particles flocculated and could be separated from the
fluid by filtration. The same experiments were performed using a Type 1 fluid.
In this case no flocculation was observed. Since the main difference between
the two types of fluid is the thickening system, the flocs should contain the
thickener and possibly also some of the additives. The infrared spectrum of the
floc separated from Type 2 fluid, is shown in figure 7. Before the spectrum was
recorded the floc was washed with water and subsequently dried at 100 °C for two
hours. The most dominant spectral features are the OH vibrations in the
hydrogen bonding region ( around 3380 (1/cm)), the methylene
stretching vibrations at 2928 (1/cm) and 2857 (1/cm), and the
carboxylate modes at 1564 (1/cm)and 1413 (1/cm). These
vibrational modes are also evident in the spectrum of LTV1704 adsorbed on
aluminium oxide, and should originate from the thickener. To remove the
thickener from Type 2 fluids, it would therefore be possible to flocculate the
thickener using e.g. 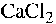 (aq), and subsequently filtrate the solution. In this
context it should be mentioned that fine particles of cement or CaCO3 seemed to
be bring about the same flocculation behaviour. The advantage of using cement
particles is that the flocs formed are precipitated at the surface of the
particles, and therefore easier to remove from the remaining fluid.
(aq), and subsequently filtrate the solution. In this
context it should be mentioned that fine particles of cement or CaCO3 seemed to
be bring about the same flocculation behaviour. The advantage of using cement
particles is that the flocs formed are precipitated at the surface of the
particles, and therefore easier to remove from the remaining fluid.
Adsorption of glycerol at 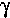 -
-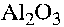 :
:
In the formulation of Type 2 fluids as well as Type 1 fluids, corrosion
inhibitors (e.g. benzotriazol and triethanolamin) are added to the fluids in
order to prevent corrosion of the aluminium surfaces. Otherwise, Al(OH)3 (gibbsite )
or 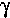 -AlO(OH) ( böhmite ) may be formed at the surface. Instead
of anti oxidizing agents it would be possible to use an agent that strongly
adheres to the aluminium surface thereby preventing its oxidation. Since it is
well known from other chemical systems that an increased number of hydroxyl
groups participating in hydrogen bonding may also dramatically increase the
stability and strength of the hydrogen bonding system, we thought of glycerol
as a suitable candidate.
-AlO(OH) ( böhmite ) may be formed at the surface. Instead
of anti oxidizing agents it would be possible to use an agent that strongly
adheres to the aluminium surface thereby preventing its oxidation. Since it is
well known from other chemical systems that an increased number of hydroxyl
groups participating in hydrogen bonding may also dramatically increase the
stability and strength of the hydrogen bonding system, we thought of glycerol
as a suitable candidate.
Experiments with 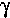 -
-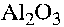 and small amounts of glycerol in water showed that only
2.5 wt-% glycerol was able to prevent the formation of bayerite. This is an
intresting result also from an environmental point of view, since glycerol is a
natural harmless substance. Therefore, the corrosion inhibitor effect of
glycerol, and also its effect on the flow properties of the fluid should be
more thoroughly investigated.
and small amounts of glycerol in water showed that only
2.5 wt-% glycerol was able to prevent the formation of bayerite. This is an
intresting result also from an environmental point of view, since glycerol is a
natural harmless substance. Therefore, the corrosion inhibitor effect of
glycerol, and also its effect on the flow properties of the fluid should be
more thoroughly investigated.
Conclusions
Based on the experiments performed, we may end up with the following conclusions and suggestions of further investigations:
* Although the thickening system may be adsorbed on 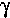 -
-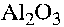 , the flocculation
of the thickener using Ca(II) ions or ions of higher charge number seems to be
a more effective and probably also a less expensive way to remove the
thickener. Adsorption on cement particles exhibited promissing results.
However, the regeneration of the particles and the influence of particle-size
distribution on adsorption and regeneration properties should become the
subject of further investigation.
, the flocculation
of the thickener using Ca(II) ions or ions of higher charge number seems to be
a more effective and probably also a less expensive way to remove the
thickener. Adsorption on cement particles exhibited promissing results.
However, the regeneration of the particles and the influence of particle-size
distribution on adsorption and regeneration properties should become the
subject of further investigation.
* The adsorption of anionic surfactants on ion exchangers containing non-charged functional groups is very strong, and may be even stronger if the functional groups are charged. Non-ionic surfactants should therefore be used, in order to increase the life-time of the ion exchanger.
* Glycerol was found to be an interesting substitut for corrosion inhibitors that are currently in use. It prevents the corrosion of aluminium oxide and is a harmless chemical often used as sweetening agent. Moreover, it may serve as an antifreeze, and deicing agent. Other potential and harmless corrosion inhibitors should be tested.
Acknowledgements
The authors want to thank Mrs Maine Ranheimer for valuable help with spectroscopic measurements and laboratory experiments. Special thanks are due to Anders Dahlgren and Peter Mattsson for discussions about recycling problems. Financial support provided by Coldtech is gratefully acknowledged.