A Field Study of Frazil Ice Accumulation and Adhesion on Trash Racks
Annika Andersson
Div. of Water Resources Engineering
Luleå University of Technology
S-951 87 Luleå, Sweden
Lars-Olof Andersson
Div. of Water Resources Engineering
Luleå University of Technology
S-951 87 Luleå, Sweden
and
SKEGA AB
S-934 81 Ersmark, Sweden
November 1992
ABSTRACT
A field experiment to study frazil ice blockage of water intake trash racks in
hydropower plants is described. The experiment, conducted during the 1990/1991
winter using a submerged video camera, was designed to investigate ice accumulation
on trash racks and compare this with laboratory tests. Differences between ice
accumulation and adhesion on steel bars with and without a rubber coating were examined.
The submerged video camera functioned well and was found to be an excellent
tool to document and evaluate these ice phenomena. Frazil ice problems occured in air temperatures
as high as -4°C. Frazil ice accumulation started on the upper section of the trash racks and progressed
downwards. Ice was mainly deposited on the upstream face of the racks. The rubber coating material,
with its poor adhesion to ice, appeared to mitigate frazil ice problems in the intakes in two ways.
Ice was much more easily removed from trash rack elements coated with rubber and frazil
did not stick to coated trash racks under small depress of supercoolings.
1. INTRODUCTION
Frazil ice is the name given to small ice particles formed in
turbulent, supercooled water. The particles are primarily discoid in shape and
supercooling of only a few hundredths of a degree is enough to favour frazil
ice formation. The particles grow as long as the water is supercooled. During
this `active' stage they easily adhere to objects with which they collide
(Carstens, 1970). Observations in the field have shown individual frazil ice
particle sizes between 1 and 15 mm (Wigle, 1970, Ashton, 1979, Osterkamp et
al., 1982).
Water velocities higher than 1 m/s cause enough turbulence to keep the frazil
ice particles well mixed (Carstens, 1970, Matousek, 1984). Layered frazil ice
and slush ice are formed when the flow velocity is between 0.6 and 1 m/s. Skim
ice and an ice cover are formed at velocities lower than 0.6 m/s.
A fundamental study of frazil ice particle formation and growth was carried
out by Daly (1984). In order to simulate the motion of frazil ice crystals, a
numerical model is under development (Svensson et al. 1988, Andersson et al.
1991).
Laboratory tests to achieve a better understanding of the prevention of frazil
ice problems have been conducted (Daly 1987a, Daly 1987b). Small scale
experiments using trash racks at field sites have been carried out (Mussalli
1987, Haynes et al., 1991).
The accumulation of frazil ice on water intakes is a serious problem in many
hydro-power plants operated in cold climates (Ashton, 1986, Daly, 1991). A
complete blockage resulting in total shutdown, preceded by a large reduction in
power production is not unusual. Production losses due to ice problems may be
costly for the hydro-power industry. At just one Swedish plant during a 10 year
period, the average cost of ice problems and heating the trash rack was 1
million SEK/year, equivalent to $0.2 million/year. The costs of having staff on
a 24-hour alert were not included.
2. OBJECTIVES
To improve the understanding of frazil ice formation and growth, a
research project was initialized in 1987 at the University of Luleå,
Sweden. The long term goal of the project is to increase the knowledge of water
intake blockage due to frazil ice and to find materials and methods that will
reduce ice adhesion to trash racks.
The aims of this study were:
a) To observe the weather conditions and temperature changes before frazil ice
problems occurred.
b) To record the frazil ice accumulation on single bars and
on the entire trash rack, with respect to water depth, flow rate and time.
c) To estimate the adhesion forces between ice and trash rack elements with
different surface materials at field sites.
We know of no earlier investigations in full scale power plants that provide
answers with regard to the influence of water depth, the single bar geometry,
side walls etc. Experiments made in the laboratory with model trash racks might
give different answers than trials with trash racks in field. The field tests
were also used to indicate the practical value and limitations of laboratory
tests. Controlled experiments in a flume located in a cold room can be
conducted and repeated. In the field, frazil ice formation is highly variable.
Conclusions drawn from results on a small scale need to be compared with real
situations in the field, in order to evaluate the practical merits of using
laboratory experiments to find the solution to the prevention of ice problems
in a hydro-power plant.
It was hoped to verify laboratory results by comparing estimates of adhesion
between ice and rubber with the adhesive forces between ice and steel
surfaces.
3. EXPERIMENTAL
Experiments were conducted during the 1990/91 winter at three
hydro-power plants in Sweden: Finnfors on the Skellefte River; Untraverket on
the River Dalälven, and Olidan on the Göta River, listed from north
to south (see locations in Figure 3.1). These three power plants were chosen
because they often experience frazil ice problems. The design of Untraverket
and Olidan permits the easy installation and maintenance of test equipment (see
figure 3.2). Each intake can be drained in a few minutes.
General data for the power-plants are shown in Table 3.1.
Table 3.1: General data for the hydro-power plants tested.
---------------------------------------------------------
Power Plant Head [m] Flow [m3/s] Power
Production
[MW]
---------------------------------------------------------
Finnfors 20.0 250 50
Untraverket 13.5 350 42
Olidan 32.0 550 135
3.1. Test equipment
The experiment was documented with a video camera, Sony CCD 500,
installed in a marine pack MPK-F340. This equipment makes recordings possible
under water down to a depth of 40 metres. An underwater lighting system,
IKELITE UVHC-3, was used to improve the light conditions. The power of the
light was 20 W which was enough to give a clear and bright picture without too
much light scatter from particles in the water. Both the marine pack and the
light were set in a steel box to avoid damage to the equipment due to impact
from timber or ice blocks. Descriptions of the test equipment for each power
plant are given below.
Finnfors, Skellefte River
At Finnfors one trash rack section (1.2 x 7.0 m) was replaced by an
identical rack coated with a 3 mm thick rubber material. The location is shown
in Figure 3.3. A qualitative evaluation, by operating the trash rack cleaner,
of different ice accumulations was achieved by comparing the performances of
the surfaces of the original and coated racks.
Untraverket, River of Dalälven
Upstream of the plant, a 1 km long canal runs between a dam and the
front of the intake. The first 700 m runs in a westsouthwest to northnortheast
direction and the last 300 m runs east to west. The canal has a width of 80 m
and a depth of 6 m. No test equipment was set up on the existing trash rack.
The video camera and its steel box were mounted on the trash rack cleaner
(Figure 3.4). The ice accumulation across the whole depth down to 8 and 9 m,
could then be recorded.
Olidan, Göta River
A trash rack rake moves up and down in front of the trash rack. The
rake is used to rinse the intakes from small objects like grass, leaves and
other trash that causes head losses. The rack has a 6 m long shaft which
permits rinsing down to 4 m below the water surface. In our experiments the
rinsing part of the rake was replaced by the steel box, housing the underwater
video camera. Consequently recordings could be done down to 4 metres.
Three steel test bars with a rectangular cross section 50 mm in depth and 30
mm wide were mounted in front of the existing, heated, trash rack (figure 3.5).
The middle bar was coated with the same rubber material as that used at
Finnfors. A rubber coated wire with a triangular cross section was mounted next
to the bars, intended to evaluate the influence on ice accretion of
self-induced vibrations combined with a rubber coating. The 60 mm spaced test
specimens were electrically insulated from the trash rack and mounted
approximately 0.1 m in front of it. This design assured that water past the
test specimen first. Heat from the control trash rack would then not affect the
ice build-up on the test bars but assured that the ice accretion started on the
test sections. In this test design, mechanical rinsing of the test section was
not possible.
Underwater video recordings showed the ice accumulation at different depths
and also the difference in ice growth on the steel bars and coated bars.
Particle behaviour in front of the rack was also recorded. Video recordings
were used to evaluate the adhesion of ice to the different specimens.
3.2. Experimental variables
3.2.1. Temperature measurements and weather conditions
The temperature was regarded as the most critical parameter in the test
and it was decided that it should be determined by as many different
independent systems as possible. The strategy included the easy replacement of
damaged of temperature probes, preferably without draining the intake. The
temperature was measured at three different depths in order to find eventual
temperature gradients during the freezing process. The different systems used
were thermistors, copper/constantan thermocouples and Pt 100 platinum
resistance thermometers. The Cu/constantan probes were used to measure water
temperature at the test site, air temperature outside the intake approximately
2 m above the water level and air temperature at the test set up, inside the
trash rack house.
Thermocouples of copper/constantan were individually shielded in polyethylene
tubes, sealed with a PVC-resin. The shielded gauges were inserted in steel
tubes, fastened on the trash racks for protection. Care was taken to place the
tubes at the required depth. Each thermocouple was individually checked at 0°C
in a mixture of ice/snow and water, before and after the field tests were
conducted. The necessary corrections were made during data analysis. It was
then possible to measure the temperature with an accuracy of a few hundredths
of a degree.
Thermistors or NTC (Negative Temperature Coefficient) resistors are non-linear
and show an exponential relationship between resistance and temperature in
R=exp(a+bT)
where T is the temperature in degrees Kelvin, R is the resistance in
ohms and a and b are constants. The exponential relationship means that the
sensor is sensitive to small changes in temperature and, since thermistors are
cheap, they are an interesting alternative to other measuring systems for
temperature, especially if the temperature has to be monitored at many
points.
Thermistors used in this test have been NTC-Resistors 60-284-50, with a
nominal resistance of 50 000 W at +25 °C supplied by ELFA, Sweden. Since the
overall resistance in the wire and connections is much smaller, only a few
ohms, the system will be fairly insensitive to changes in resistance in the
wire due to temperature changes. Each thermistor was soldered to filmshielded
wires in a KAHL 55-752-04 cable from the same supplier. The two conductors were
individually insulated with rubber tape and placed inside a degreased
polyethylene tube with an inside diameter of 4 mm. The thermistor glass bead
was centred and the PE-tube subsequently filled with a two component epoxy
resin. After the epoxy had been allowed to cure, the PE-tube was slowly dipped
into and withdrawn from a PVC-resin twice to form an approximately 1 mm thick
watertight layer. A junction box in which the resistance was converted to a
voltage proportional to the temperature was constructed. The voltage thus
obtained was then used as an input to the data logger.
Pt 100 temperature probes, with an accuracy of 1/10 DIN, shielded by stainless
steel tubes were used during calibration at the field site. Using a Thermolyzer
S2541 between the Pt-probe and the data logger, the required resolution was
achieved in the vicinity of 0 °C.
Finnfors. Thermocouples were used to record water and air temperature.
The water temperature was measured 2 m below the water surface and 0.5 m behind
the trash rack.
Untraverket. Air and water temperature were measured with
thermocouples. The water temperature was measured continuously at a water depth
of 6 m and on some occasions at water depths of 4 and 8 m. Once a day, at 7:30
a.m., the air temperature and water temperature were recorded manually with an
accurate mercury thermometer and a Pt-100 Platinum resistance thermometer
respectively.
Synoptic and automatic weather stations are located around Sweden. Data on air
temperatures, amount of precipitation, wind speeds and wind directions were
obtained from stations near to each power plant.
3.2.2. Water velocity
Finnfors. Water velocities were measured at 10 m intervals on
straight lines perpendicular to the intake at three locations 50 m upstream of
the power plant. At the time of measurement, the river was partly covered with
ice.
Untranverket. The water velocity profile across the entire trash rack
was measured when no frazil ice was present and the flow rate through the plant
was normal for the season.
4. RESULTS
4.1. Temperature measurements and weather conditions
Finnfors. Frazil ice formation appeared suddenly at Finnfors on
13 November 1990. Temperatures recorded before and after the frazil problems
occurred are tabulated in Table 4.1. Head losses larger than 2 m exposed the
temperature gauge to the air which caused the peak shown in Figure 4.1.
Table 4.1: Temperatures observed at Finnfors power plant.
---------------------------------------------------------
Date Time T (water) °C T (air) °C
---------------------------------------------------------
901106 15:30 1.04 - 9
901107 07:30 0.77
901112 07:30 0.18 - 6
15:30 0.27 - 2
901113 07:30 0.08 - 8
15:30 0.03 - 8
901114 07:30 0.01 - 14
15:30 0.04 - 14
901115 07:30 0.01 - 8
15:30 0.02
901116 07:30 0.04 - 1
15:30 0.03
Untraverket. Figure 4.2 shows air temperatures. At Gävle and Films
Kyrkby the average daily temperatures were measured in the automatic and
synoptic station. The third curve represents the temperature recorded at the
power plant at 7:30 each morning. In general all three temperature curves
follow the same pattern.
Air and water temperatures recorded each morning at Untraverket power plant
from November 1 to the end of December, are shown in Figure 4.3. The water
temperature declined while air temperatures were below 0 °C. Air temperatures
fluctuated more rapidly than water temperatures. Water temperature did not vary
with depth.
Frazil ice problems occurred during December,1990, coincident with snowy
weather (Figure 4.3). The wind direction was northnortheast to northnorthwest
during the first part of December, i.e. parallel in the downstream direction to
the intake canal, and during the rest of the month the wind direction was
southwest. The average wind speed was 3.5 m/s.
It should be noted that air temperatures of only -4 °C were sufficiently low
to cause frazil ice problems (see Dec 5 1990 in figure 4.3).
Olidan. Olidan experienced frazil ice problems during February 1991.
Average daily air temperatures were measured at a nearby weather station
(Såtenäs, figure 4.4). Frazil ice problems occurred on the February
5 at an air temperature of -7.0 °C. Little precipitation occurred during that
day and the average wind speed was 5 m/s from the northeast.
4.2. Water velocity
Finnfors. The water velocities, measured upstream of the intake,
ranged between approximately 0.3 m/s at the centre of the river, to 0.8 m/s
about 20 m from the shore, figure 4.5.
Untraverket. Immediately upstream of the intake, the velocity profile
was constant with depth except close to the surface and bottom, see figure 4.6.
The velocity at the surface was low (0.2 m/s) because of the skimmer wall which
extended 1.5 m below water level, i.e. 2.5 m below the top of the trash rack
(Figure 3.2). A wake was caused that affected the flow in front of the rack.
Below the skimmer wall, the water velocity reached a constant value of 1.2 m/s.
Upstream of the intake canal, the water velocity varied between 0.2 and 0.6 m/s
depending on the flow through the plant (100 and 300 m3/s respectively).
Olidan. The water velocity was not measured but was calculated to 0.8
m/s when the flow rate was 40 m3/s.
4.3. Frazil ice accretion
Untraverket. Untraverket experienced frazil ice problems several
times at the beginning of the 1990/91 winter season. The freezing process under
water was recorded on two different occasions on a third occasion, the gate
upstream of the intake was lowered to the bottom and the entire trash rack was
drained and exposed in the trash rack house. The frazil ice blockage and the
thickness of the accumulated ice was estimated. Figures 4.7 and 4.8 show the
results of ice accretion measurements on two of the trash rack bars on two
occasions. These were considered representative of other samples. Figure 4.7
indicates that ice accumulation started at a depth of approximately 2 m. 1 hour
and 20 minutes later, ice had reached to a depth of almost 7 m at intake 2 and
approximately 5 m at intakes 3 and 5.
Figure 4.8 indicates that on this occasion the ice build-up had been in
progress for a longer time than that shown in the previous figure. The ice
growth reached a depth of 7 m in the middle of the day and to the bottom, 8m,
at approximately 17.00, resulting in complete blockage.
The bars were visible through the ice accumulation. The ice remained
transparent, but became more opaque when complete blockage occurred. This can
be explained by a high porosity of the accumulated ice. During accumulation the
deposited ice became more and more dense, and hence increasingly opaque. The
accumulated ice thickness was 10 to 15 cm immediately before the intake was
drained on 21 December.
During this winter period, water was discharged through the spillways
resulting in energy production losses. Figure 4.9 shows the spillage during the
period when frazil ice problems occurred. The spillage peak on 9 December
occurred because of drifting ice problems not because of frazil ice. The plant
turbine had to shut down to enable the intake to be cleaned from surface ice,
transported from upstream. The remaining peaks (marked with arrows) were caused
by frazil ice problems.
Olidan. A video, recorded while the hydro-power plant was in operation,
showed the ice accumulation at different depths and also the differences in ice
growth between the steel bars and coated bars. Particle behaviour in front of
the rack was also recorded. Olidan experienced frazil ice problems on the night
of 4/5 February. Ice accumulation on only the uppermost 1 m of the rectangular
steel bars of the test section could be recorded because a large amount of
block-ice from upstream collected above most of the rack (see figure 4.10). The
ice blocks, together with frazil ice deposition, caused a head loss of
approximately 2.5 m, rather than complete blockage. The ice blocks originated
from the break up of the ice sheet on the river, which is kept open for
navigation.
Frazil ice accumulated on the steel bars to a depth of 1 m with an ice
thickness of 5 cm. Ice samples were taken from both the test bar and the block
ice. Thin sections were prepared and evaluated in cross-polarized light. The
block-ice consisted of frazil ice. The size of the individual frazil ice
particles in the blocks ranged between 5 and 8 mm. The trash rack was drained
and exposed to warm air and the ice blockage could be recorded. Frazil ice
problems continued on several occasions over a period of approximately 2
weeks.
4.4. Frazil ice adhesion
Finnfors. At 00.30 on November 14, the engineer on duty was
informed by the Power company's Central Control Board that power production was
very low at Finnfors. When he arrived at the power station, the head loss was
14 m.
No difference in accumulated ice thickness on the original trash rack and the
coated test rack was observed before the cleaning. The flow patterns in front
of both trash racks were also similar. It should be noted that the trash rack
cleaner measured 3 m in width, but the test section was only 1.2 m wide. The
rack cleaner therefore covered a further 0.9 m, on each side of the non-coated
trash racks, during cleaning.
A significant difference in the de-icing performance of the rinsing equipment
was observed when the rubber coated trash rack was cleaned, compared to the
original trash rack elements. Ice was removed from the coated rack in large
pieces (approximately 1 by 1.2 m). This effect was observed several times.
After cleaning, a substantially greater flow was indicated through the rubber
coated trash rack than through the control rack, despite a reduced spacing
between the trash rack bars of approximately 8% due to the coating. Ice removed
from the un-coated rack was fragmented. The problem continued for about 24
hours until all ice was mechanically removed and full production could be
achieved.
Untraverket. Recordings taken during the drainage of the intake on
December 21, showed the difficulties involved in the removal of ice from the
conventional steel trash rack using the trash rack cleaner. Although the trash
rack was exposed to temperatures of approximately 10 °C, the ice was difficult
to remove. Half of the trash rack area was equipped with electrical heating
which caused the ice to melt and fall off after some time.
Olidan. No ice accumulated on the bar coated with rubber material. The
rubber coated test wire did not accumulate ice either. Whether this was due to
self induced vibrations or to the rubber material itself is not clear. Ice on
the original trash rack was difficult to remove shortly after it had been
drained and exposed to warmer air.
5. DISCUSSION
Frazil ice accumulation as a function of depth was the main parameter
studied at Untraverket. The video recordings indicated that the ice
accumulation started at the upper part of the trash rack and progressed
downwards. Frazil ice started to accumulate approximately 2 m below the water
surface. This can be explained by the location of the skimmer wall which
reached 1.5 m below the water line and caused wake currents and low velocities
at the upper part of the trash rack. Below 1.5 m depth the velocity was
constant and the frazil ice particles could be transported directly towards the
trash rack, to form the first ice growth.
Several reasons may account for the origin of ice accumulation in the upper
region of the trash racks. Frazil ice is more concentrated close to the water
surface than at a depth below the surface. When the upper section of the trash
rack becomes blocked, water carry-ing the frazil ice particles is forced
downwards. The effect on pressure of the freezing water may contribute to a
small extent to the phenomenon. A change in pressure equivalent to a 10 m
increase in water depth causes a change of -0.0074 °C (Hobbs, 1974) in the
freezing point. Depths of this magnitude are common in front of an intake and
the supercooling is not more than a few hundredths of a degree.
Ice on the trash racks is composed mainly of frazil ice particles, carried by
the water and deposited on the bars. The trash racks in this study were all
located indoors where the temperature was well above freezing point (around 10°C), so that ice growth due to the transfer of latent heat from the rack to the
air will not take place. Calculations of the magnitude of this form of ice
growth have shown that, even when the air temperature is below freezing point,
the mass growth rate is negligible compared with the mass rate of deposition of
the frazil ice suspended in the flow (Daly, 1987a). Growth resulting from the
transfer of the latent heat to the supercooled water is 100 times greater than
growth due to latent heat transferred from the rack to the air. This is still
negligible compared with deposited frazil ice.
Video recordings showed that the accumulated ice was highly porous, i.e. had a
high water content which caused transparency. Adhesive strength between the ice
and the steel bar was nevertheless high. This was corroborated by the inability
of the trash rack cleaner to remove the ice.
Frazil ice may not be the only ice problem in a hydro-power plant such as
Olidan. Navigation on the river produced a large amount of block ice that
originated from broken ice cover. Ice blocks together with frazil ice caused
blockages of the entire intake.
The video recordings showed frazil ice particles very clearly. The discoid
particles which moved irregularly due to turbulence, could be seen when they
reflected light. Particle sizes up to 8 mm indicated that they have been
transported long way in supercooled water.
In 1990, severe frazil ice problems occurred at Finnfors, in the early morning
of November 14, coincident with supercooling down to -0.05 °C, recorded with
the acquisition system. A high level of supercooling may favour adhesion, since
the bars will cool to a lower temperature relative to the freezing point (Daly,
1987a).
Table 5.1: Thermal conductivities for different materials (1*Hands
et.al., 1977, 2*Broberg et.al., 1976, 3*Weast et.al., 1984, 4*Hobbs, 1974)
------------------------------------------------------------------------
Material 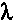 Material
Material 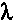 (W/mK) (W/mK)
------------------------------------------------------------------------
Iron (Fe) 80 1* Carbon black filled 0.33 3*
(60 phr) rubber
Stainless steel, 15 2* Water 0.56 4*
SS-2333
Copper 401 1* Ice (solid) 2.2 4*
Rubber (pure gum) 0.18 3*
(W/mK) (W/mK)
------------------------------------------------------------------------
Iron (Fe) 80 1* Carbon black filled 0.33 3*
(60 phr) rubber
Stainless steel, 15 2* Water 0.56 4*
SS-2333
Copper 401 1* Ice (solid) 2.2 4*
Rubber (pure gum) 0.18 3*
Thermal conductivity values (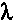 -values) for different materials are listed in
table 5.1. Value given have been determined at 0 °C except for SS-2333 which
was measured at +20 °C. The conductivity should be important since a criterion
for ice build up is that the latent heat can be transported away from the
surface to which the ice attaches.
-values) for different materials are listed in
table 5.1. Value given have been determined at 0 °C except for SS-2333 which
was measured at +20 °C. The conductivity should be important since a criterion
for ice build up is that the latent heat can be transported away from the
surface to which the ice attaches.
A difference in performance between materials of different thermal
conductivities has been observed at Olidan. Submerged copper and a steel plates
are used as frazil ice indicators.
In Olidan, no frazil ice accumulated on the rubber coated specimen. This was
not expected since no difference in ice accretion between the tested materials
has been observed in laboratory experiments (Jensen et al., 1989). At Finnfors
no difference in accumulated ice thickness was observed after the icing had
taken place. The probable reason for this discrepancy may be a combination of
three factors.
Firstly, the supercooling at Finnfors was approximately -0.05 °C. The channel
has a large width-to-depth ratio which results in a large cooling rate it being
regarded as one of the coldest reaches in Sweden. In Olidan, frazil ice
problems occurred at only one intake and they were not severe. A maximum head
loss of about 2 m was recorded.
Secondly, the depth of the clearance between the test specimen, extension in
the flow direction, was greater in Finnfors (120 mm) than in Olidan (50 mm).
Hence, the probability for an ice particle to attach should be greater at
Finnfors. A possible explanation of the time lag in Figure 4.1 could be that
the rubber coated part stayed open for a longer time than the uncoated part. If
this was the case, an increased water velocity, due to a decreased open area
during the ice accumulation, would further prevent the ice particles from
adhering to the coated bars. Unfortunately, the icing process could not be
documented below the water surface.
Finally the lower accretion of ice to rubber may be explained by the lower
surface energy of rubber, approximately 30 mJm-2 compared with metals, that
usually show values of hundreds of mJm-2 in practice, due to natural
contamination (clean machined metal surfaces have surface energies of 1000 to
3000 mJm-2). If the adhesive forces are small enough, the drag force may be
high enough to remove the deposited ice before bridging occurs.
A significant difference in ice adhesion between rubber-coated trash racks and
non-coated trash racks was expected. The choice of rubber material, designated
P1-13534, in the field test was based on materials tested in the laboratory
which had earlier shown low ice adhesion properties (Andersson, 1989). It was
however modified to comply with the requirements of tear strength, wear
resistance, good adhesion to metals etc. Video inspection at the coated rack
element one year after the installation showed minimal or no wear of the rubber
surface, despite the coated trash rack. Due to the coating extending 3 mm in
front of the adjacent trash rack, elements have been subjected to extra high
strains.
It should also be emphasised that materials that show low ice adhesion values
do not automatically show a resistance to ice accretion. Ice attached to the
rubber coated trash rack was however removed much more easily than ice from the
non-coated trash racks, even though the test section measured only 1/3 of the
width of the mechanical rake device. If the test section had been de-iced
separately, the difference might have been even more pronounced.
Earlier investigations (Sayward, 1977, Andersson, 1989) have shown that
adhesion forces always exist between ice and substrate since all surfaces have
a certain surface energy. Although these forces are small for polymers, ice may
well be difficult to remove from such surfaces. In the case of trash racks, an
optimum solution to blockage due to frazil formation would be that, as the
frazil ice builds up, the reduction in section area would build up a pressure
that at a specific level would shear the accumulated ice away. This is what we
think happened in Olidan. This tendency has also been found for this material
in laboratory tests (not published). Beside pure shear stresses, the ice
build-up may disturb the normal flow pattern across the rack and create
vibrations. Personnel at the power plants explain that the trash racks
sometimes start to "sing" during the frazil ice build-up. This extra energy
input may be enough in combination with a low energy coating to allow for "self
rinsing". If this is not enough, then mechanical rinsing is required.
By carefully designing the trash rack elements and selecting the proper
surface treatment many of the problems that exist today could be to a great
extent reduced.
There is, as far as we can see, no simple solution to icing on submerged
structures.
6. CONCLUSIONS
Conclusions that can be drawn based on the field experiments are:
* The frazil ice accretion on trash rack starts at the upper section,
progresses downward and ends in a complete blockage.
* The ice adhesion is lower on rubber coated materials than on steel.
* In one test, frazil ice accumulated on the test steel bar but not on the
rubber coated test bar.
* A submerged video camera is a very powerful tool to register frazil ice
formation at field sites.
7. FURTHER WORK
Further work should include the following:
* Repeat the recordings of ice accumulations on the trash rack at Untraverket.
Try to capture the start and the course of the ice blockage as well as the
drainage on the same occasion. Analyse statistics on the course of frazil ice
problems on intake trash rack.
* Improve and extend the test equipment in Olidan. Build a system to record
frazil ice accretion on a single steel bar and compare this with laboratory
experiments.
* Record temperature data and weather conditions that correspond to frazil ice
problems.
* Replace a larger section of the trash rack with a coated section to be able
to operate the trash rack cleaner within the coated section at Finnfors.
Evaluate the effect of the trash rack cleaner on the coated section.
* Extend the field test to instal coated trash rack sections in other power
plants.
8. ACKNOWLEDGEMENTS
Special thanks are due to Dr. Lennart Billfalk for his advice and for
reviewing this report. Sincere thanks are extended to the personnel at the
electric plants of Finnfors, Untraverket and Olidan. The work has been financed
by SKEGA AB, Swedish State Power Board, Stockholm Energy, Skellefteå
Kraft, The Swedish National Industrial Board, Coldtech and Luleå
University of Technology.
9. REFERENCES
Andersson, A. and Svensson, U. (1991) A Numerical Simulation of
an Ice Particle Trajectory. In Proceedings at the 4th International Phoenics
Users Conference, Miami Beach, USA, pp. 241-288.
Andersson, L.O., (1989). Ice Adhesion to Polymer Materials. Proceedings at
the 10th International POAC Conference, Luleå, Sweden, pp.786-795.
Ashton, G.D. (1979) Frazil Ice. American Scientist.
Ashton, G.D. Ed (1986) River and Lake Ice Engineering. Littleton,
Colorado: Water Resources Publications. ISBN 0-918334-59-4.
Ashton, G.D. (1988) Intake Design for Ice Conditions. Developments in
Hydraulic Engineering-5 (P. Novak, Ed.). New York: Elsevier Applied
Science.
Broberg, B. et al (1976) Formelsamling i hållfasthetslära.
Publication no. 104. Department of solid Mechanics, Royal University of
Technology, Stockholm. Printed in Stockholm, pp 186-193.
Carstens, T. (1970) Heat Exchanges and Frazil Formation. Proceedings of the
Symposium on Ice and its Action on Hydraulic Structures, Reykjavik, Island,
IAHR Paper no. 2.11.
Daly, S.F. (1984) Frazil Ice Dynamics. CRREL Monograph 84-1, US
Army CRREL, Hanover, NH.
Daly, S.F. (1987 a) Modelling trash rack freeze up by frazil ice.
Proceedings of the first International Symposium on Cold Regions Heat
Transfer, June 4-7, Edmonton, Alberta.
Daly, S.F. (1987 b) The evolution of frazil ice in rivers and streams.
Research and Control. Proceedings of the first International Symposium on
Cold Regions Heat Transfer, June 4-7, Edmonton, Alberta.
Daly, S.F. (1991) Frazil Ice Blockage of Intake Trash Racks. Cold Regions
Technical Digest, No. 91-1, March, US Army CRREL, Hanover, NH.
Hands, D. and Horsfall, F. (1977) Thermal Diffusivity and Conductivity of
Natural Rubber Compounds. RAPRA Members Report No. 9. The Rubber and
Plastics Research Association.
Haynes, F.D.,Daly, S.F., Garfield, D.E. and Clark, C.H. (1991) Field Test of
Trash Rack Heating to Prevent Frazil Ice Blockage. Preliminary Report,
US Army CRREL, Hanover, NH.
Hobbs, P.V. (1974) Ice Physics. Printed in Great Britain by J.W.
Arrowsmith Ltd, Bristol Bs3 2NT, England. ISBN 0-19-851936-2, p357.
Jensen, H. (1989) Testing of ice growth on models of bars for trash racks.
Report No. STF60 F89047. Norwegian Hydrotechnical Laboratory, Trondheim,
NORWAY.
Matousek, V. (1984) Types of Ice Run and Conditions for their Formation. In
Proceedings at IAHR Ice Symp., Hamburg, Vol I, pp. 315-328.
Mussalli, Y.G., Gordon L.S. and Daly, S.F. (1987) Frazil ice control using
electromechanical vibrators and ice resistance coating. Presented at ASCE
WATERPOWER 87, Portland, Oregon, August 19-21.
Osterkamp, T.E. and Gosink, J.P. (1982) Selected Aspects of Frazil Ice
Formation and Ice Cover Development in Turbulent Streams. Proceedings of the
Workshop (2nd) on Hydraulics of Ice-Covered Rivers, NRCC Associate
Committee on Hydrology and Subcommittee on Hydraulics of Ice-Covered Rivers,
Civil Engineering Department, University of Alberta, Edmonton, Alberta T6G 2G7,
pp. 131-146. (Also see discussions and replies, p. 147)
Svensson, U. and Andersson, A. (1988) A Numerical Model of Ice Accretion on
Structure, Proceedings at IAHR Ice Symposium, Sapporo, Japan, Vol. 2,
pp. 436-445.
Weast, R.C., Astle, M.J. and Beyer, W.H. (1984) CRC Handbook of Chemistry
and Physics. 64th edition. Library of Congress Card No. 13-11056. Printed
in U.S.A. ISBN 0-8494-0464-4, pp E9-E10.
Wigle, T.E. (1970) Investigation into Frazil, Bottom Ice and Surface Ice
Formation in the Niagra River. Proceedings of the Symposium on Ice and its
Action on Hydraulic Structures, Reykjavik, Island, IAHR Paper no. 2.8.
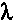 Material
Material  (W/mK) (W/mK)
------------------------------------------------------------------------
Iron (Fe) 80 1* Carbon black filled 0.33 3*
(60 phr) rubber
Stainless steel, 15 2* Water 0.56 4*
SS-2333
Copper 401 1* Ice (solid) 2.2 4*
Rubber (pure gum) 0.18 3*
(W/mK) (W/mK)
------------------------------------------------------------------------
Iron (Fe) 80 1* Carbon black filled 0.33 3*
(60 phr) rubber
Stainless steel, 15 2* Water 0.56 4*
SS-2333
Copper 401 1* Ice (solid) 2.2 4*
Rubber (pure gum) 0.18 3*